Glowing Bouncy Egg Experiment
What could be better than making a naked bouncing egg?! Making a glowing one, of course! This bouncy egg experiment teaches little scientists about egg anatomy and osmosis and takes just a few minutes to set up. It seriously egg-citing!
And speaking of excitement, our 30 Science Experiments are kid-approved and loaded with fun!

Getting Ready
To prep, I first gathered my supplies:
- An egg (one for each highlighter color)
- Clear glass
- Vinegar
- Highlighters
Bouncy Egg Experiment
Making a glowing bouncy egg is surprisingly simple – the hardest part is having to wait.
To start, we took the polyester cylinder out of the highlighter and squeezed it to extract as much of the ink as we could. The easiest way was to use our fingers, so be prepared for a little mess.

Next, my 3 year-old, Q, very carefully added a raw egg to each of the glasses. Then, we covered them with white vinegar. The pink and orange solutions were very bright, but the solution with the yellow highlighter turned almost clear when the vinegar was added.

Now that the bouncy egg experiment was underway, all we had to do was wait. We could see bubbles forming on the surface of the egg almost immediately, but we had to wait a couple of days to see the real results.
Two days later, we checked on our eggs and found that the shells had disintegrated. He gently rubbed the shell with his fingers to reveal the membrane below.

Q was really curious about the egg’s membrane and where it was located in a raw egg. So, I cracked an egg on a plate and showed him that the membrane is normally stuck right on the inside of the shell.
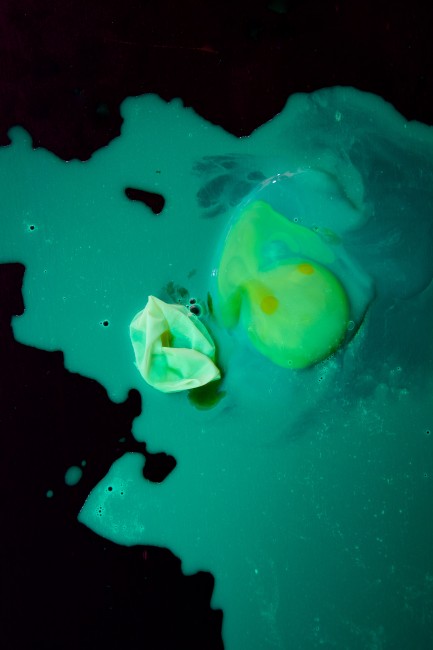
I rinsed the other eggs under the sink and passed them to Q for some bouncing. I brought out the black light to see how the highlighter affected the egg.

We were surprised that the pink and orange highlighters didn’t make the eggs glow. The yellow highlighter, which we thought wouldn’t work when it turned clear, glowed a bright green.

After squishing and squeezing each egg, Q couldn’t wait to bounce them. He discovered really quickly that the eggs will break if thrown hard enough!
You can see the yolk is still intact and the membrane that helped the rubbery egg bounce is laying next to the yolk. Q was pretty sad he popped all three of his eggs. But since this bouncy egg experiment is so simple to set up, it took just a few minutes to get a new bouncy egg started again.
The Science Behind It
When you leave the egg in vinegar, the acetic acid in the vinegar breaks down the calcium carbonate shell, producing the tiny carbon dioxide gas bubbles you see.
Once the egg shell dissolves, the egg expands slightly because the membrane is semi-permeable. That means it allows some things to pass through it. This process is called osmosis.
Some of the water with the highlighter ink passes through the membrane into the egg and causes it to swell and glow. In the picture below, the first 3 eggs have their shell removed and the last egg is a regular raw egg.

Osmosis is the movement of a liquid, like water, across a membrane. Membranes like to be balanced on both sides. The vinegar solution is mostly water with only a little vinegar and ink in it, while inside the membrane is protein with a little water. So, the glowing water molecules travel from the vinegar into the egg to try to balance the concentrations. The egg expands and glows!
More Simple Science with Major “Wow!” Factor
Want to step up your science game?! Download 30 of our favorite science experiments PLUS a corresponding science journal for young scientists!